Checklist for CSSD for NABH preparation and its quality indicators
Checklist for CSSD for NABH preparation and its quality indicators
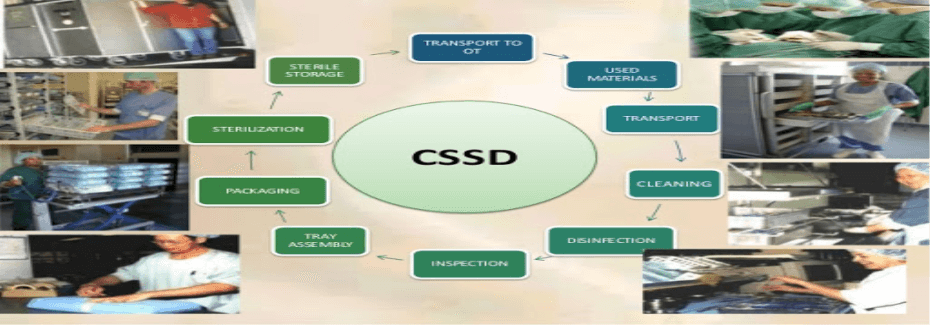
Central Sterilization and Supplies Department (CSSD), has its importance in prevention of cross infection in hospitals. An effective functioning of CSSD will reduce the HAI traceable to use of instruments on patients’ care. For this CSSD must follow standard practices in its sterilization and supplies operations. NABH recommends following standards to be implemented in CSSD.
Infrastructure
• The CSSD should be located in a delineated area where there is less or no external traffic movement.
• Location of CSSD should be either close to OT or should be connected to OT with safe & quick transfer mechanism, like dumbwaiters.
• Entry to CSSD should be restricted to only staff working in CSSD
• The CSSD layout should have well demarcated zones, which includes
o Collection zone (or soiled zone) where the soiled and used items should be received and sorted
o Cleaning zone where washing, cleaning and packaging of items should be done
o Sterilization zone where the actual sterilization of packages should be done
o Storage – This can be considered a part of sterilization zone, where sterilized packs are stored till its distribution
• The zones must lead to unidirectional movement of people and supplies.
• Entry to sterile zone should be after taking necessary infection control precautions such as hand washing, wearing of gowns/aprons, gloves etc.
• The sterilization zone (specially storage) should have a higher air pressure to prevent outside air to enter in this area
• Emergency exit route should be identified and displayed
• Easy access to fire fighting measures (like extinguisher, or hose pipe) should be available
• Equipment: CSSD should have required sterilization and cleaning equipment in well-functioning condition. Although, not explicitly specified in standards but it is expected that CSSD must have technologically sound equipment. Also, equipment should be relevant to the type of materials required to be sterilized. For eg., if rubber tubes have to be sterilized ETO should be available. Horizontal autoclave with two doors is preferable than vertical single door type.
Processes:
• SOP should be documented for each activity done in CSSD. These activities include
o Procedure of cleaning
o Procedure of packing
o Procedure of disinfection
o Procedure of sterilization (separate SOP for each type of sterilization equipment)
o Procedure of storage and issue
o Safety precautions and guidelines
• A policy should be there on reusable devices/items which specifies following
o List of items that can be re-used (items not in list should automatically be considered, non-reusable)
o Number of times it can be re-used
o On whom can it be re-used (like on same patient or on different patient)
o Processing required before reuse of the items
• Sterilization equipment must be calibrated at a regular interval. A preventive maintenance checklist should also be available for each equipment
• Each sterilization equipment should have an identification number, which should be displayed on the equipment
• Each pack, that is being sterilized must be labelled with following information
o Date of sterilization
o Date of expiry
o Equipment number in which it was sterilized
o Load number in which it was loaded for sterilization
• Record of each load sterilized in CSSD should be maintained. The record must contain
o Date
o Load number
o Description of contents that were included
o Temperature, pressure and time-record chart
• Validation tests must be done in CSSD. The validation tests must include
o Physical/Chemical test – should be done for each load
o Biological spore test – at-least weekly basis for each equipment
• The CSSD must maintain record of all validation test reports
• A procedure of recalling items should be there in case of sterilization breakdown.
• List of hazardous chemicals in CSSD should be available
• MSDS of each hazardous chemical should be available
Staff knowledge
• Staff working in CSSD must have knowledge of following
o Infection prevention practices to be followed in CSSD
o Zones and unidirectional movement
o Hand hygiene practices and other standard precautions
o Validation tests done in CSSD
o When sterilization breakdown should be considered
o Procedure to recall when sterilization breakdown occurs
o Relevant staff should know their work SOP
o Occupational health hazards of working in CSSD
o Hazardous chemicals and its spill management
o Other general topics like, employee rights, use of fire extinguisher, emergency codes etc.
• Staff in patient care areas who receive CSSD supplies must know following
o About the labels on sterile packs, specially expiry date
o Storage condition that should be maintained
o Condition under which sterile packs must not be used
o What to do in case a sterile pack has been recalled
o How to send soiled/used items
Quality Indicators for CSSD
• % of HAI happening due to instrument/devices used on patients
• Number of times of sterilization failure
• % re-sterilization required due to improper storage
• % of non-compliance to sterilization practices